Blog Archives
Reformulated gasoline
Hello everyone!!
Today I am going to talk about one of the several improvements gasoline has gone by, in order to adapt it to the development of fuels.
In the 90’s, several law enforcements took place in the United States, encouraging gas producers to reduce the pollution of it. Oxygenate use increased substantially with the start of the federal oxygenated fuel program (for controlling CO) in 1992. There was a great expectancy with this reformulated gasoline, which aimed to reduce gas pollution levels. It was blended in order to, reduce Volatile Organic Compounds and toxic emissions of conventional gasoline. However, its parameter values were in the same range of the normal type. So we could conclude that it was definitely not a new gasoline.
The main differences between reformulated (RFG) and conventional gasoline were the following ones
-The Reid Vapor Pressure level,wich is a common measure of the volatility of gasoline. It was reduced in RFG only in summer, and it is the main responsible for the reduction of Volatile Orgainc Compounds in RFG.
-Reduction of benzene levels, a human carcinogen.
-In RFG Methyl Tertiary-Butyl Ether (MTBE) and ethanol (EtOH) represent the majority of oxygenate use. Oxygenates are usually employed as gasoline additives to reduce carbon monoxide that is created during the burning of the fuel.
-By the way, auto producers supported RFG because of the smaller variabaility in the quality of gas, implying a lower probability for bad gas.
-As it has been tested, these new features shouldn’t reduce vehicle’s performance.
In the following graphic, it is shown how RFG didn’t imply a revolution in the use of gas, in spite of the light improvement on the poluting agents.
|
|
Conventional gasoline |
RFG |
|||
|
|
Average |
Range2 |
Average |
||
| RVP3 | 8.7-S | 6.9-15.1 | 7.2/8.1-S | ||
| (psi) | 11.5-W | 11.5-W | |||
| T50 (øF) |
207 | 141-251 | 202 | ||
| T90 (øF) |
332 | 286-369 | 316 | ||
| Aromatics (vol%) |
28.6 | 6.1-52.2 | 23.4 | ||
| Olefins (vol%) |
10.8 | 0.4-29.9 | 8.2 | ||
| Benzene (vol%) |
1.60 | 0.1-5.18 | 1.0 (1.3 max) |
||
| Sulfur (ppm) |
338 | 10-1170 | 302 (500 max) |
||
| MTBE4 (vol%) |
— | 0.1-13.8 | 11 (7.8-15) |
||
| EtOH4 (vol%) |
— | 0.1-10.4 | 5.7 (4.3-10) |
||
Fuels (II) – The kerosene
Today we are introducing a new element that can be obtained from petroleum distillation, the kerosene, a thin and clear liquid formed from hydrocarbons. This transparent liquid has an intermediate density between petrol and diesel densities. At first, it was used to supply paraffin lamps with the needed energy to light. Nowadays, it is used to power jet-engined aircrafts.

This fuel is perfect for aviation because of its thermal stability during storage, its good lubrication, cleaning and volatility rate, and because it is not corrosive. Furthermore, it freezes at – 40 degrees, what makes kerosene the perfect combustible to work at high altitudes and low temperatures. It is mainly used to power turbines of airplanes.
A gas turbine is a driving machine that transforms the energy from combustion into mechanical energy in the shape of a stream of air at high pressure and temperature. This energy is used to move a drive mechanism like an airplane propeller.
The heat released in the combustion when the fuel ignites depends on the quality of the fuel. When the volumetric energy (energy per unit volume) increases, the stored energy in the tanks of the aircraft also increases. Therefore, the planes’s autonomy raises up.

Fuels (I) – The gasoline
Hello everyone!
As I promised in my last post, I am back to talk about a petrol product, the gasoline.
There’s no denying that gasoline is of paramount importance. Indeed, those internal combustion engines my colleague Rodrigo talks about in his last post work thanks to it.
-We pointed out gasoline was obtained refining petrol, but of course that’s not the only process gasoline experiences till it ends in petrol stations. Straight-run gasoline is a mixture of hydrocarbons with between 4 and 12 carbon atoms per molecule.
-Gasoline is highly volatile, more than diesel oil or kerosene. Its volatility is controlled blending it with butane, which boils at 0 ºC more or less at atmospheric pressure. It’s determined by a test called Reid Vapor Pressure [RVP]. Volatility is crucial for the resultant fuel. A consequence of a non-correct volatility gasoline is that in cold weather, cars fail to start.
Nevertheless, excessive volatility has drawbacks too. It may imply a vapor lock: combustion doesn’t take place because the liquid fuel has turned into gaseous and renders the fuel pump ineffective. Most countries simply have summer and winter volatility limits.
-In order to assure its stability, gasoline should be stored in an airtight container, to prevent oxidation or water vapors mixing, and at a stable cool temperature, to reduce the chance of the container leaking. If not, gums and solids may appear resulting in “stale fuel”, hampering engines to start. Here we have an average gasoline container.
-Density of gasoline rounds 719.7 kg. Gasoline floats on water, water cannot usually extinguish a gasoline fire.
Paradoxically, gasoline is not fuel’s main component. What’s more, it’s not even 20% of an average fuel. That’s because it doesn’t meet nowadays engines’ requirements, specially the octane rating [RON], what measures gasoline’s resistance to autoignition. Commercial fuels vary between 90 and 100 RON, depending on the country.
To fill this lack and some more, other petrol products with higher RON are added, such as
-Reformate gasoline, produced in a catalytic reformer with a high octane rating and high aromatic content, and very low olefins (alkenes).
-Cat cracked gasoline produced from a catalytic cracker, with a moderate octane rating and high olefins(alkene) content.
-Hydrocrackate, alkylate, isomerate and others are included to increase fuel’s performance.
–Lead additives modify the autoignite behavior of gasoline in high-compression internal combustion engines, so lead based additives were usually added to fuel. Nonetheless, it was discovered how environmentally harmful lead is and its use is steadily decreasing.
Once explained its main features, an obvious question overcomes us. How does fuel provides us with energy? Well it’s obtained from its combustion, and as our several Chemistry teachers have taught us, that’s the conversion of a hydrocarbon to carbon dioxide and water.
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
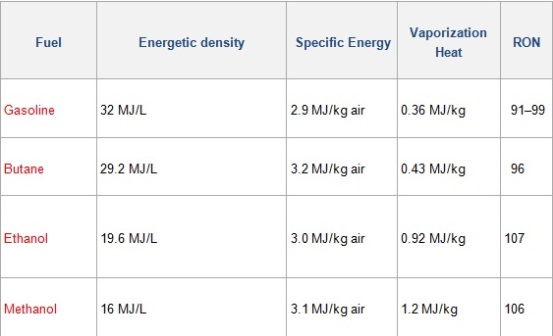
Gasoline contains about 35 MJ/L (Check the enthalpies seen in class, there’s no comparison!). Gasoline blends differ, and the actual energy content by up to 4% more or less than the average. Here we have a graphic showing the different energy density of gasoline and its blends.
Well, once understood the breathtaking rates of energy gasoline provides us with, why so much bad press against gasoline? Why are governments so obsessed with reducing the amount of cars in highways?
That’s because combustion of a liter of gasoline produces 2,33 kg of carbon dioxide, a greenhouse gas. Moreover, ecologists complain about how harmful extraction and refining processes are.
Well, that’s what in general terms we need to know about gasoline in order to understand better its functions and its uses.
Thanks for reading it and hope it served its purpose!